LC-MS for RNA Therapeutics to Ensure Product Quality and Process Consistency
41:21
LC-MS for RNA Therapeutics to Ensure Product Quality and Process Consistency
Share
Related Videos
In Webinars Home
-
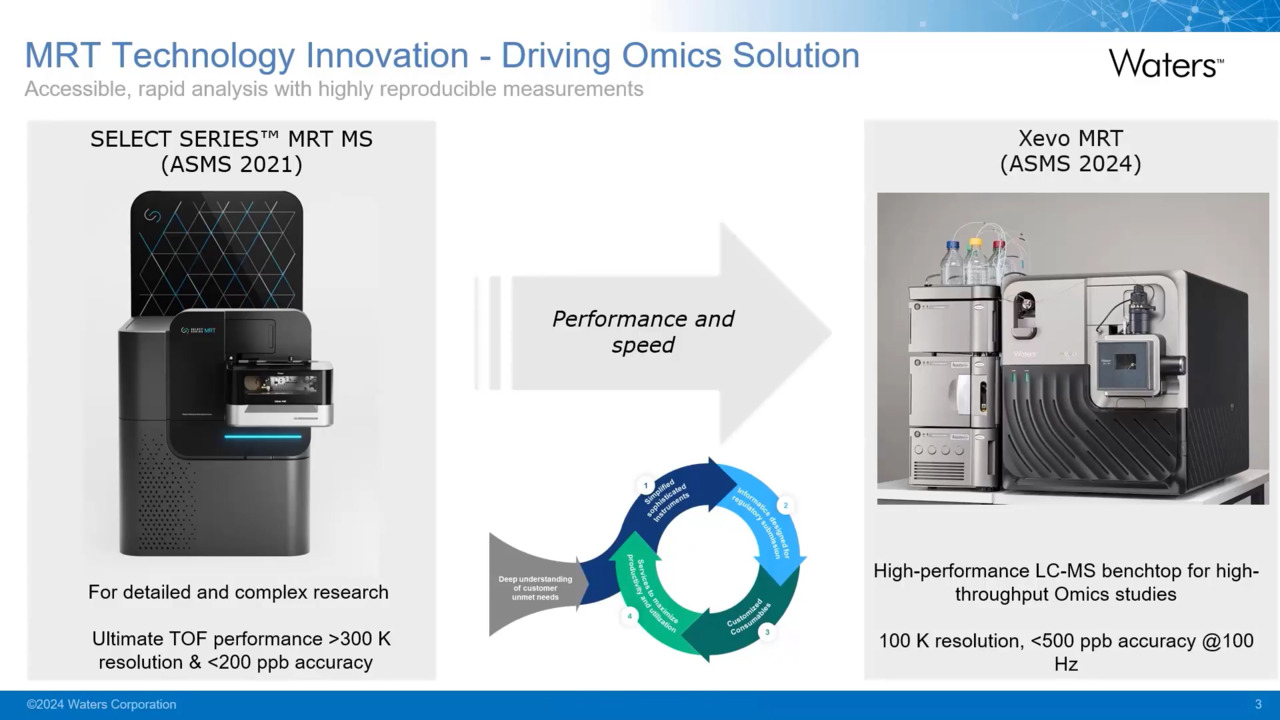
Play video Xevo MRT MS Digital Launch : Pushing the boundaries of science with multi reflecting time-of-flight technology
Xevo MRT MS Digital Launch : Pushing the boundaries of science with multi reflecting time-of-flight technology
Discover the Xevo MRT MS: Performance and Speed Without Compromise. Join Prof. Perdita Barran from the University of Manchester as she discusses her Parkinson’s Disease research and insights from our development team on this incredible technology.
57:26
-
Play video Metabolomics Profiling of Sebum and Serum to Monitor Progression Markers of Parkinson's Disease
Metabolomics Profiling of Sebum and Serum to Monitor Progression Markers of Parkinson's Disease
Join world-renowned Professor Perdita Barran from the Institute of Biotechnology as she shares her incredible research into Parkinson's Disease.
22:49
-
Play video Streamlined mAb Subunit LC-MS Workflow for Multiple Attribute Monitoring of Biosimilar mAb Candidates During Bioprocessing and Development
Streamlined mAb Subunit LC-MS Workflow for Multiple Attribute Monitoring of Biosimilar mAb Candidates During Bioprocessing and Development
Clone selection, early in the development lifecycle, has an outsize impact on the ability to match the quality target product profile (QTPP) for a given product.
38:53
-
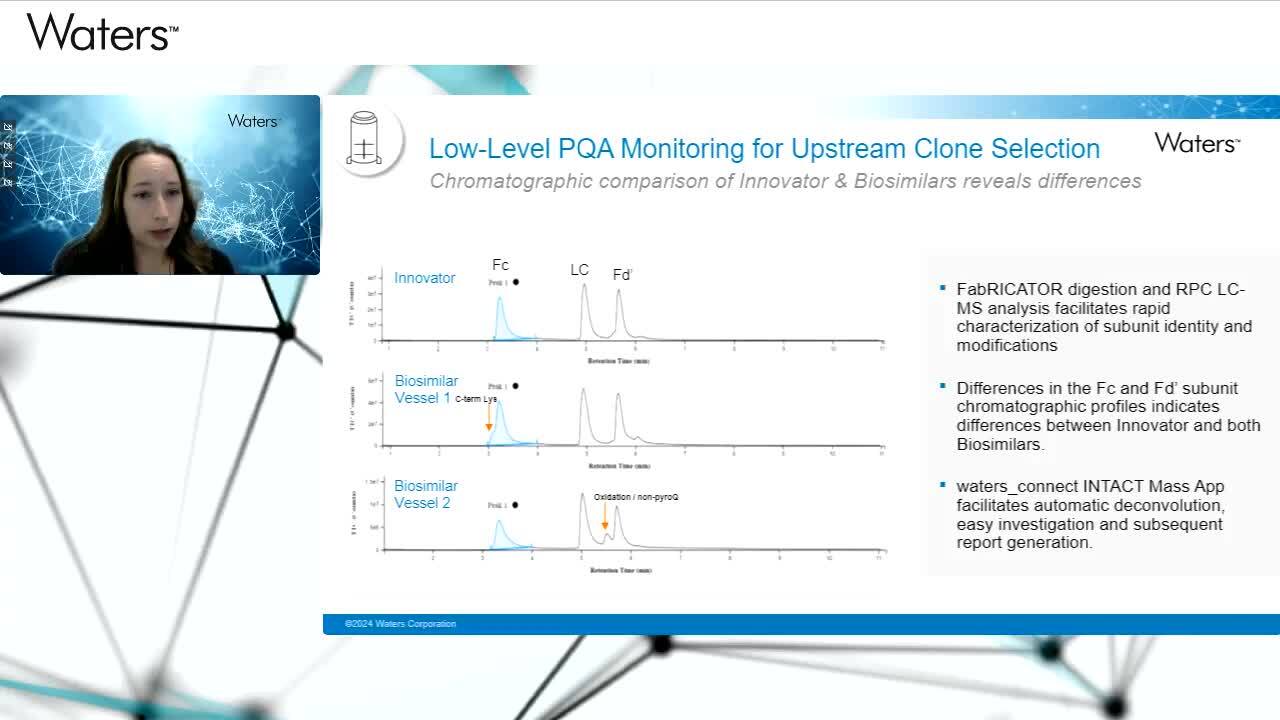
Play video Robust Multi-Level LC-MS Workflows for Biosimilar Comparability Assessment
Robust Multi-Level LC-MS Workflows for Biosimilar Comparability Assessment
Watch this presentation to learn about the uses of the Xevo G3 QTof mass spectrometer in a case study of biosimilar mAb characterization at intact, subunit, and peptide level.
48:24
-
Play video PFAS Analysis by EPA 1633 | Building a Comprehensive Capability
PFAS Analysis by EPA 1633 | Building a Comprehensive Capability
Join Waters Corporation for an insightful webinar on PFAS Analysis by EPA 1633 using LC-MS/MS. Learn essential techniques, best practices, and future advancements in PFAS testing to elevate your lab's capabilities.
44:12
-
Play video Automated LC-MS Workflow for CQA Assessment of Protein Therapeutics with Speed and Simplicity for Process Development
Automated LC-MS Workflow for CQA Assessment of Protein Therapeutics with Speed and Simplicity for Process Development
Here we demonstrate how sample prep has been greatly simplified and automated to enable rapid LC-MS (intact and subunit) analysis at higher throughput, with less human intervention, all while providing high quality to support process development.
1:01:40